Special protein found in bacteria could cause cancer cells to self-destruct, Spanish scientists say
A protein emitted by a specific type of bacteria can cause cancer cells to self-destruct, potentially paving the way for new treatments, according to an international team led by Spanish scientists.
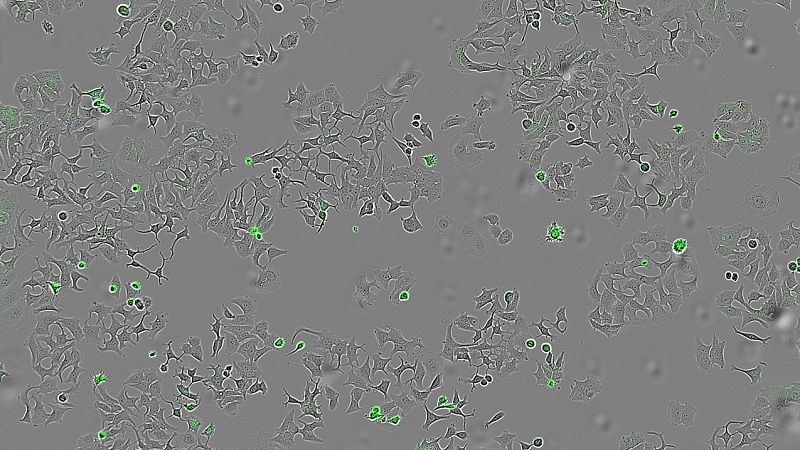
The promising protein, known as HapA, is secreted by the bacterium Vibrio cholerae, according to the study, which was published in the journal Cell Death Discovery. Researchers tested the new mechanism in breast, colon, and pancreatic tumour cells.
The HapA protein acts uniquely on two key receptors – PAR-1 and PAR-2 – which are found on the surface of tumour cells and are normally associated with tumour progression, inflammation, and blood clotting.
HapA cleaves them at sites that are different from those recognised by human enzymes that break down proteins. That triggers a rapid process leading to apoptosis, or programmed cancer cell death.
"This work demonstrates the potential of bacterial proteins as anti-tumour therapeutic tools," Antonio Hurtado, a researcher at the University of Salamanca in Spain, said in a statement.
The team demonstrated that HapA specifically caused cancer cell death by conducting experiments with mutant strains of V. cholerae that lacked this protein, as well as another type of bacteria that had been genetically modified to produce only HapA. In all cases, only the presence of HapA resulted in reduced tumour cell viability.
They also tested whether HapA was effective against different types of tumours by applying a liquid containing all the proteins secreted by the bacteria to breast, colon, and pancreatic cancer cell lines.
"We wanted to see if human cells of different tumour types were still alive and could multiply after being in contact with these bacterial substances, in particular with the HapA protein," Hurtado said.
These findings open promising avenues for the development of new cancer treatments and underscore how studying pathogens can reveal unexpected molecular mechanisms with therapeutic potential.
The researchers said HapA's ability to selectively activate the PAR-1 and PAR-2 receptors via a cleavage site that is different from human enzymes suggests that cell signalling could be adjusted in a targeted way, helping to avoid side effects.
Today

